Biosimilar
to Humira® Adalimumab


Biosimilar
to Rituxan® Rituximab


Biosimilar
to Stelara® Ustekinumab


Biosimilar
to Herceptin® Trastuzumab


Biosimilar
to Lucentis® Ranibizumab


Biosimilar
to Avastin® Bevacizumab


Biosimilar
to Neupogen® Filgrastim


Biosimilar
to Eylea® Aflibercept


Biosimilar
to Soliris® Eculizumab
Where our adalimumab biosimilar* is approved:
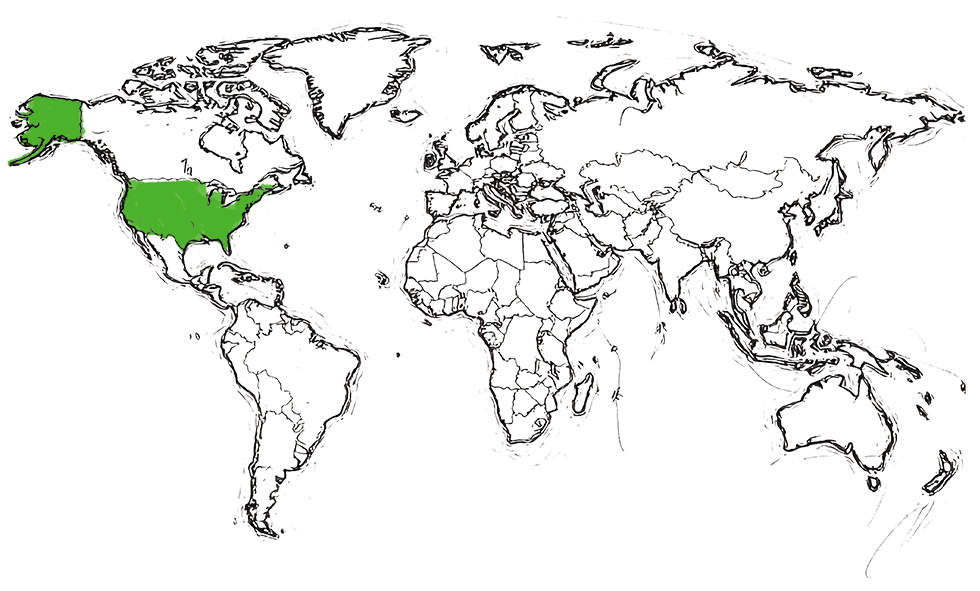
Approved by FDA (US)
in 2024
| Biosimilar to Humira® (adalimumab) | ||
|---|---|---|
| Teva product information | Where approved | |
| SIMLANDI® | FDA SITE | United States |
In collaboration with Alvotech in the United States.
FDA=Food and Drug Administration.
All trademarks are the property of their respective owners.
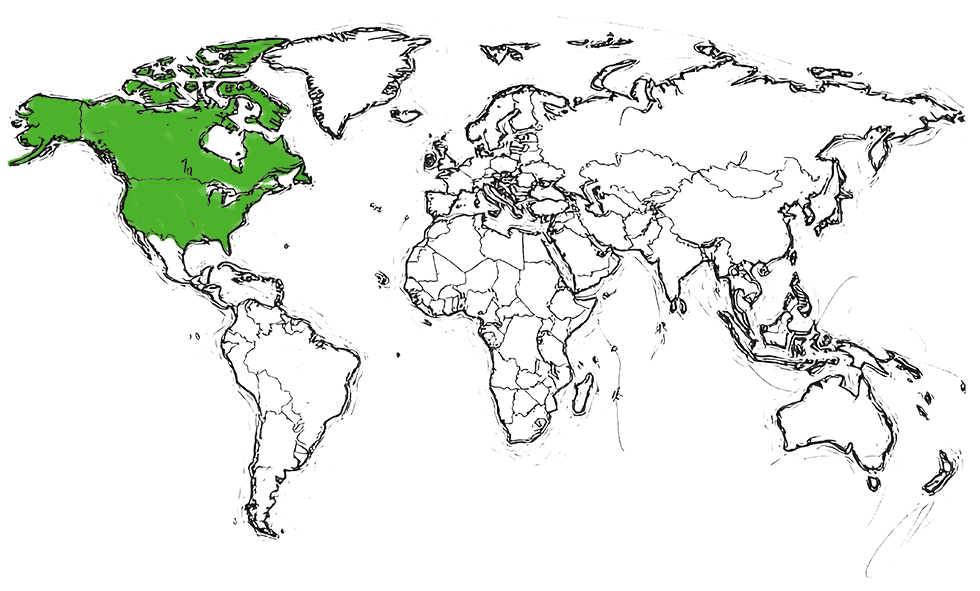
Approved by FDA (US)
in 2018
Approved by Health Canada
in 2019
| Biosimilar to Rituxan® (rituximab) | ||
|---|---|---|
| Teva product information | Where approved | |
| TRUXIMA® | FDA SITE | United States |
| TRUXIMA® | HEALTH CANADA SITE | Canada |
In collaboration with Celltrion Inc in the United States and Canada.
FDA=Food and Drug Administration.
All trademarks are the property of their respective owners.

Approved by FDA (US)
in 2024
| Biosimilar to Stelara® (ustekinumab) | ||
|---|---|---|
| Teva product information | Where approved | |
| SELARSDITM | FDA SITE | United States |
In collaboration with Alvotech in the United States.
FDA=Food and Drug Administration.
All trademarks are the property of their respective owners.

Approved by FDA (US)
in 2018
Approved by Health Canada
in 2019
| Biosimilar to Herceptin® (trastuzumab) | ||
|---|---|---|
| Teva product information | Where approved | |
| HERZUMA® | FDA SITE | United States |
| HERZUMA® | HEALTH CANADA SITE | Canada |
In collaboration with Celltrion Inc in the United States and Canada.
FDA=Food and Drug Administration.
All trademarks are the property of their respective owners.
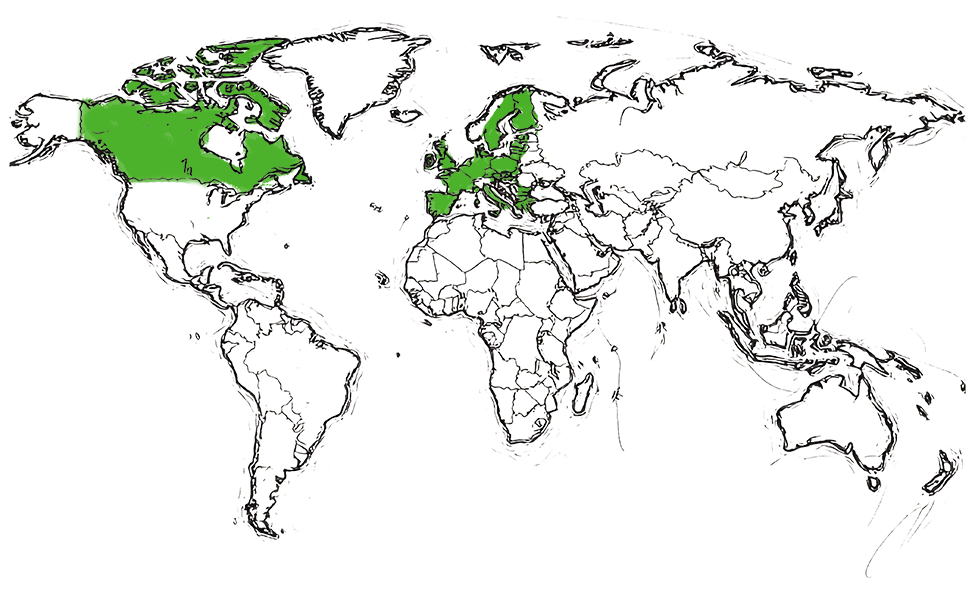
Approved by Health Canada
in 2022
Approved by MHRA (UK)
in 2022
Approved by EMA (EU)
in 2023
| Biosimilar to Lucentis® (ranibizumab) | ||
|---|---|---|
| Teva product information | Where approved | |
| RANIVISIO® | EMA SITE | Europe |
| RANOPTO® | HEALTH CANADA SITE | Canada |
| ONGAVIA® | MHRA SITE | United Kingdom |
In collaboration with Bioeq AG in the European Union, Canada, and the United Kingdom.
EMA=European Medicines Agency; MHRA=Medicines and Healthcare Products Regulatory Agency.
All trademarks are the property of their respective owners.
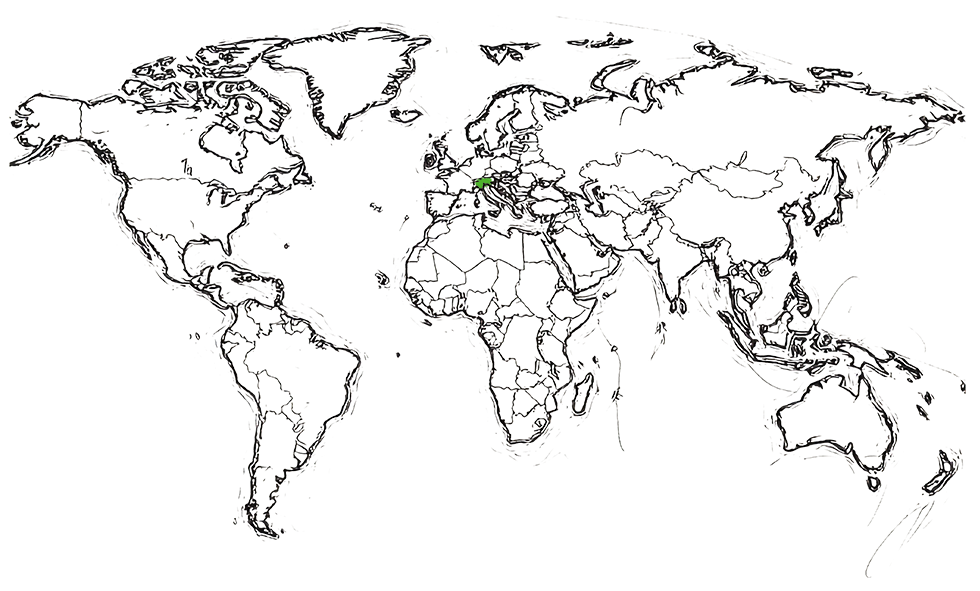
Approved by Swissmedic (CH)
in 2021
| Biosimilar to Avastin® (bevacizumab) | ||
|---|---|---|
| Teva product information | Where approved | |
| BEVACIZUMAB-TEVA® | SWISSMEDIC SITE | Switzerland |
In collaboration with mAbxience in Switzerland.
All trademarks are the property of their respective owners.
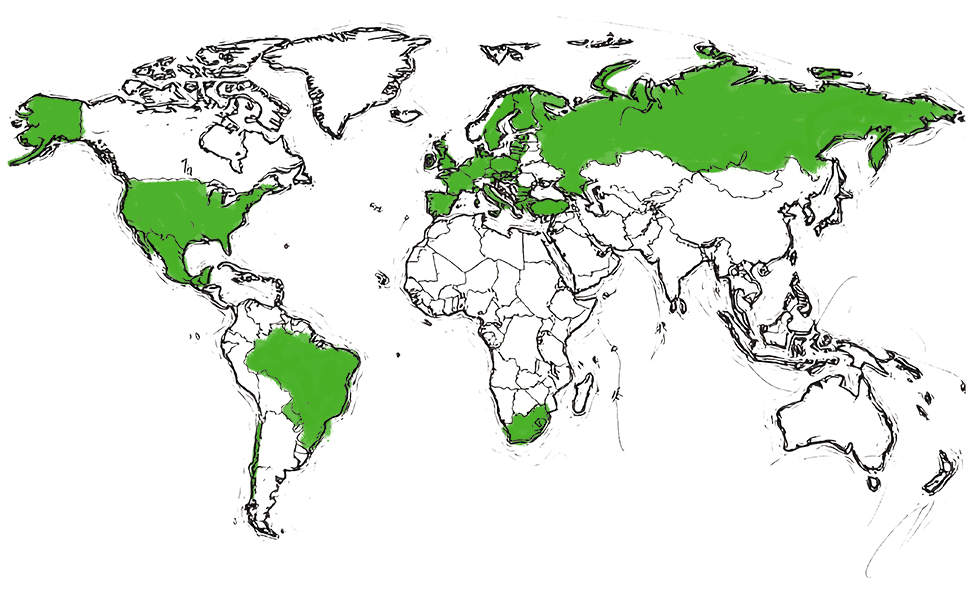
Approved by FDA (US)
in 2018
Approved by EMA (EU)
in 2008
Approved by MINZDRAV (RU)
in 2010
Approved by COFEPRIS (MX)
in 2023
Approved by TMMDA (TR)
in 2015
Approved by ANVISA (BR)
in 2010
Approved by IL MOH (IL)
in 2009
Approved by DINAVISA (PY)
in 2024
Approved by ISP (CL)
2014
Approved by SAHPRA (ZA)
in 2017
| Biosimilar to Neupogen® (filgrastim) | ||
|---|---|---|
| Teva product information | Where approved | |
| TEVAGRASTIM® | EMA SITE | Europe |
| TEVAGRASTIM® | IL MOH SITE | Israel |
| GRANIX®† | FDA SITE | United States |
| TEVAGRASTIM® | ANVISA SITE | Brazil |
| TEVAGRASTIM® | MINZDRAV SITE | Russia |
| INMUNEF® | COFEPRIS SITE | Mexico |
| TEVAGRASTIM® | ISP SITE | Chile |
| TEVAGRASTIM® | TMMDA SITE | Turkey |
| Filgrastim Teva® | SAHPRA SITE | South Africa |
| TEVAGRASTIM® | DINAVISA SITE | Paraguay |
*The EMA is the only agency that classifies filgrastim as a biosimilar. In other regions, filgrastim from Teva is considered a generic.
†GRANIX is not considered a biosimilar in the United States.
ANVISA=Agência Nacional de Vigilância Sanitária; COFEPRIS=Comisión Federal para la Protección contra Riesgos Sanitarios; DINAVISA=Dirección Nacional de Vigilancia Sanitaria Paraguay; EMA=European Medicines Agency; FDA=Food and Drug Administration; ISP=Instituto de Salud Pública; MINZDRAV=Ministry of Health of the Russian Federation; SAHPRA=South African Products Regulatory Authority; TMMDA=Turkish Medicines and Medical Devices Agency.
All trademarks are the property of their respective owners.
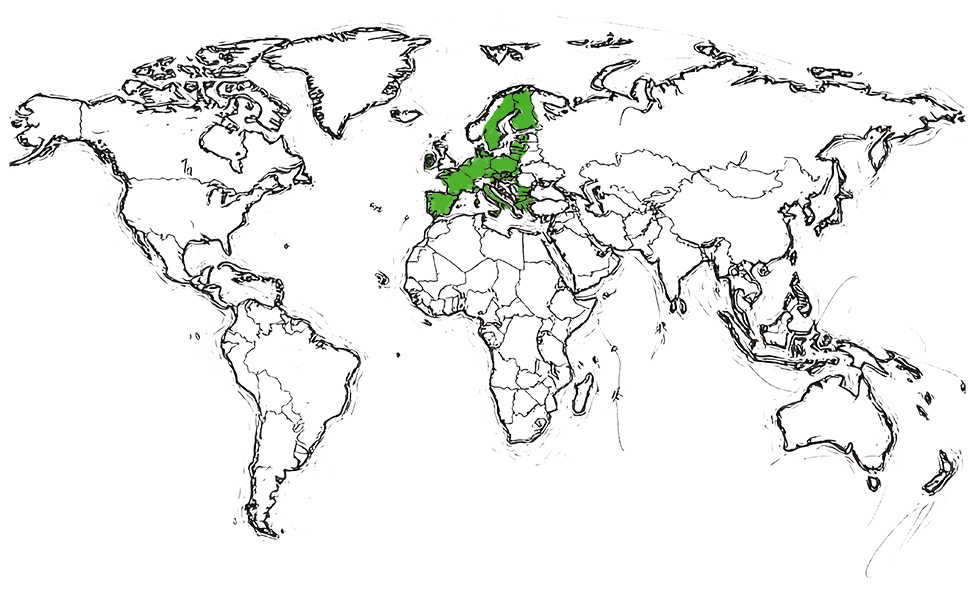
Approved by EMA (EU)
in 2008
| Biosimilar to Eylea® (aflibercept) | ||
|---|---|---|
| Teva product information | Where approved | |
| AHZANTIVE® | EMA SITE | Europe |
In collaboration with Klinge Biopharma GmbH and Formycon AG in the EEA (excluding Italy), the United Kingdom, Switzerland, and Israel (to be submitted in Switzerland and Israel).
EEA=European Economic Area; EMA=European Medicines Agency.
All trademarks are the property of their respective owners.

Approved by FDA (US)
in 2024
| Biosimilar to Soliris® (eculizumab) | ||
|---|---|---|
| Teva product information | Where approved | |
| EPYSQLI® | FDA SITE | United States |
In collaboration with Samsung Bioepis in the United States.
FDA=Food and Drug Administration.
All trademarks are the property of their respective owners.